上海金畔生物科技有限公司代理AAT Bioquest荧光染料全线产品,欢迎访问AAT Bioquest荧光染料官网了解更多信息。
TAQuest FAST qPCR Master Mix with Helixyte Green *低ROX*
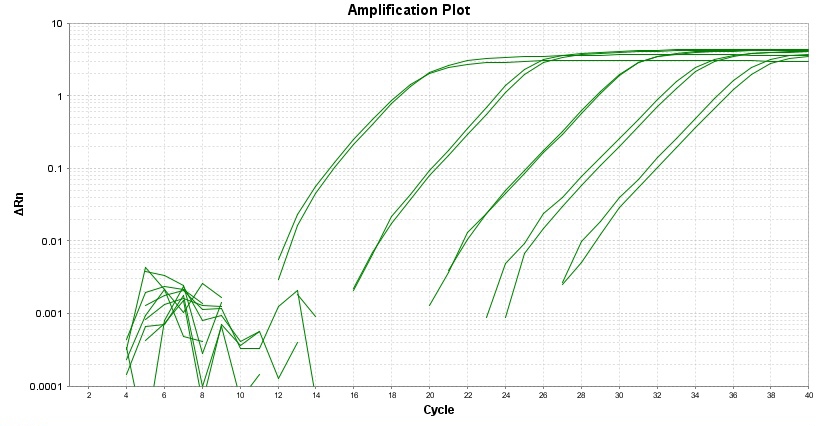 |
货号 | 17279 | 存储条件 | 在零下15度以下保存, 避免光照 |
| 规格 | 5 mL | 价格 | 3612 | |
| Ex (nm) | Em (nm) | |||
| 分子量 | 溶剂 | Water | ||
| 产品详细介绍 | ||||
简要概述
产品基本信息
货号:17279
产品名称:TAQuest FAST qPCR Master Mix with Helixyte Green *低ROX*
规格:5ml
储存条件:-15℃避光防潮
保质期:12个月
产品物理化学光谱特性
溶剂:水
产品介绍
TAQuest FAST qPCR Master Mix with Helixyte Green 是一种即用型 2X溶液,针对 qPCR 和两步法 RT-qPCR 进行了优化。对于 20 uL 反应体积中的 40 个 PCR 循环,预混液可在 50 分钟内提供结果。该混合物包括含有 dNTP 的优化缓冲液和我们专有的 TAQuest FAST 热启动 Taq DNA 聚合酶,该酶旨在允许即时热启动,最大限度地减少非特异性产物的形成,从而允许室温反应设置。运行所需的 PCR 反应只需要模板和目标引物。 TAQuest FAST qPCR Master Mix 与 Helixyte Green 确保 PCR 特异性和灵敏度对所有样品类型,如基因组、质粒、病毒和 cDNA 模板。 Helixyte Green 嵌入染料无需使用序列特异性探针即可快速检测和分析 DNA。该预混液含少量ROX 参考染料。金畔生物是AAT Bioquest的中国代理商,为您提供最优质的TAQuest FAST qPCR Master Mix with Helixyte Green *低ROX*。
适用仪器
| qPCR | |
| 仪器规格 | SYBR Green 滤波片 |
样品实验方案
注意 在室温下用 Helixyte Green *低ROX* 解冻 TAQuest™ FAST qPCR Master Mix。 使用前彻底涡旋 qPCR Master Mix。
1. 制备表 1 所示的下列反应混合物之一。
2. 轻轻涡旋混合试剂,然后短暂离心。
3. 在 qPCR 仪器中设置板并按表 2 所示操作。
表 1. 各反应每孔试剂组成
| 成分 | 体积 (25 µL/reaction) | 体积 (50 µL/reaction) | 最终浓度 |
| TAQuest qPCR Master Mix with Helixyte Green *低 ROX* | 12.5 µL | 25 µL | 1X |
| 上游引物,10 µM | 0.25-2.5 µL | 0.5-5.0 µL | 0.1-1.0 µM |
| 下游引物,10 µM | 0.25-2.5 µL | 0.5-5.0 µL | 0.1-1.0 µM |
| DNA模板 | 1-5 µL | 1-5 µL | 优化的浓度 |
| 无核酸酶水 | 25 µL | 50 µL |
表 2. 热循环参数
| 范围 | 聚合酶激活 | PCR (30-40个循环) | ||
| Hold | 变性 | 退火 | 延伸 | |
| 温度 | 95 °C | 95 °C | 55-65 °C | 68-72 °C |
| 时间 (m:ss) | 0:20 | 0:30 | 1:00 | 1:00 |
参考文献
Fatal systemic toxoplasmosis in a 3-month-old young tibetan goat (Capra hircus).
Authors: Pavone, Silvia and Crotti, Silvia and Cruciani, Deborah and D’Avino, Nicoletta and Zema, Jacopo and Morelli, Simone and Gobbi, Marco and Madeo, Laura
Journal: BMC veterinary research (2020): 423
Development of four PCR-based methods to differentiate tilefish species (Branchiostegus japonicus and B. albus).
Authors: Kang, Tae Sun
Journal: Food chemistry (2019): 1-8
A multi-screening Fast qPCR approach to the identification of abortive agents in ruminants.
Authors: Sebastiani, Carla and Curcio, Ludovica and Ciullo, Marcella and Cruciani, Deborah and Crotti, Silvia and Pesca, Cristina and Torricelli, Martina and Sebastianelli, Martina and Felici, Andrea and Biagetti, Massimo
Journal: Journal of microbiological methods (2018): 12-17
A rapid real-time PCR method to differentiate between mottled skate (Beringraja pulchra) and other skate and ray species.
Authors: Kim, Mi-Ra and Kwon, Kisung and Jung, Yoo-Kyung and Kang, Tae Sun
Journal: Food chemistry (2018): 112-119
Development of a Sensitive Real-Time Fast-qPCR Based on SYBR® Green for Detection and Quantification of Chicken Parvovirus (ChPV).
Authors: Nuñez, Luis F and Santander-Parra, Silvana H and Chaible, Lucas and De la Torre, David I and Buim, Marcos R and Murakami, Alexandre and Zaidan Dagli, Maria Lucia and Astolfi-Ferreira, Claudete S and Piantino Ferreira, Antonio J
Journal: Veterinary sciences (2018)
Evaluation and utilization of preassembled frozen commercial fast real-time qPCR master mixes for detection of cytomegalovirus and BK virus.
Authors: Glover, William A and Atienza, Ederlyn E and Nesbitt, Shannon and Kim, Woo J and Castor, Jared and Cook, Linda and Jerome, Keith R
Journal: Journal of medical virology (2016): 115-9
Fast quantitative PCR, locked nucleic acid probes and reduced volume reactions are effective tools for detecting Batrachochytrium dendrobatidis DNA.
Authors: Ruthig, Gregory R and Deridder, Benjamin P
Journal: Diseases of aquatic organisms (2012): 249-53
Multi-platform comparison of ten commercial master mixes for probe-based real-time polymerase chain reaction detection of bioterrorism threat agents for surge preparedness.
Authors: Buzard, Gregory S and Baker, Daniel and Wolcott, Mark J and Norwood, David A and Dauphin, Leslie A
Journal: Forensic science international (2012): 292-7
Real-time quantitative PCR and fast QPCR have similar sensitivity and accuracy with HIV cDNA late reverse transcripts and 2-LTR circles.
Authors: Yoder, Kristine E and Fishel, Richard
Journal: Journal of virological methods (2008): 253-6
Detection of equine herpesvirus-1 in nasal swabs of horses by quantitative real-time PCR.
Authors: Perkins, G A and Goodman, L B and Dubovi, E J and Kim, S G and Osterrieder, N
Journal: Journal of veterinary internal medicine: 1234-8
